noble gas configuration of copper|Noble gas configuration (video) : Tuguegarao The symbol of the noble gas is placed in brackets, in this case ["Ar"], which represents the electron configuration of atoms of the noble gas. The atoms of the .
Our website contains a list of credible top 10 online casinos in El Salvador with a real money prize for any taste. This list is compiled by our professionals according to various criteria, like payments security, banking, user reviews, ratings and diversity of games.
PH0 · physical chemistry
PH1 · Noble gas configuration (video)
PH2 · Noble gas
PH3 · Noble Gas Configuration
PH4 · How do you write the noble
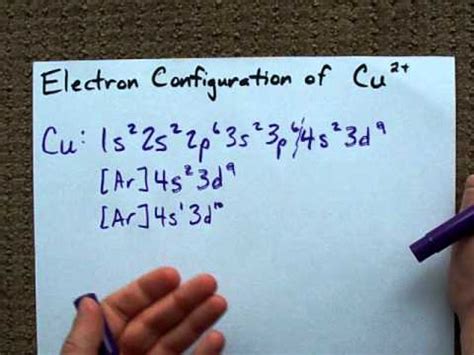
PH5 · Electron Configuration for Cu, Cu+, and Cu2+ (Copper and
PH6 · Electron Configuration for Copper (Cu, Cu+, Cu2+)
PH7 · Electron Configuration Chart of All Elements (Full Chart)
PH8 · 5.20: Noble Gas Configuration
Dhankesarilive.com - Get updates about Dhankesari Daily Results, Nagaland State Lottery, Dhankesari Coupon, Sikkim State Lotteries Result, Shillong Teer Hit Number, Kerala State Lottery Results etc. Skip to content. . Lottery Sambad Result [10.10.2023/8:00PM] Today [Dear Lottery] | ডিয়ার লটারি 8:00 টার .
noble gas configuration of copper*******Copper has an electron configuration of [Ar] 3dX10 4sX1 [ A r] 3 d X 10 4 s X 1. Now sometimes the noble state is written as [Ar] 3dX10 4sX1 [ A r] 3 d X 10 4 s X 1 or as [Ar] 4sX2 3dX9 [ A r] 4 s X 2 3 d X 9. Now the first noble state seems to be the same .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Copper .noble gas configuration of copper Noble gas configuration (video) In writing the electron configuration for Copper the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Copper go in the 2s .A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. . Answer link. (Ar)3d^104s^1 = Copper to write E.C. easily, first thing you need to do is to find the atomic number for an element (ex.Gallium [Ga]) [=] [1] Ga has . The symbol of the noble gas is placed in brackets, in this case ["Ar"], which represents the electron configuration of atoms of the noble gas. The atoms of the . These are our P electrons because they're in P orbitals, and then once we're through our 2p6 electrons, we go to 3s2 and we have two more electrons, so it's 3p2. So that's the electron configuration for silicon. Now, we can write it out using noble .Copper: Zinc: Gallium: Germanium: Arsenic: Selenium: Bromine: Krypton: Rubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: .
To write the configuration for the Copper ions, first we need to write the electron configuration for just Copper (Cu). We first need to find the number of . Filling Transition Metal Orbitals. The electron configuration for the first row transition metals consists of 4s and 3d subshells with an argon (noble gas) core. This only applies to the first row transition metals, adjustments will be necessary when writing the electron configuration for the other rows of transition metals. The noble gas before the .In practice, chemists simplify the notation by using a bracketed noble gas symbol to represent the configuration of the noble gas from the preceding row because all the orbitals in a noble gas are filled. For example, [Ne] .noble gas configuration of copper To find the electron configuration of oxygen: Look at the periodic table and find an atomic number of oxygen, which is 8. Fill these 8 electrons in the following order: 1s, 2s, and then 2p. Write the complete .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2).For example, [Ar]4s23d8 would be entered as [Ar]4s^23d^8. Give the ground-state electron configuration for copper (Cu) using noble-gas shorthand. Express your answer in condensed form as a series of orbitals. For example, [Ar]4 s 23 d 8 would be entered as [Ar]4s^23d^8. Here’s the best way to solve it. 99% (168 ratings) Share Share. [Ar ..
Such is the case with copper and its loss of one 4s electron to the 3d sub-orbital to form the noble gas configuration of [Ar]4s 1 3d 10. Manganese, on the other hand, has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 5 and a noble gas configuration of [Ar]4s 2 3d 5, resulting in one unpaired electron in each 3d sub-orbital .The same rule will apply to transition metals when forming ions. You should note that the ns electrons are always lost before the (n-1)d when forming cations for transition metals.For example, the electron configuration for Zn: [Ar]4s 2 3d 10 . the electron configuration for Zn +2: [Ar]3d 10 . The transition metals still do not end up being isoelectronic with a .They are helium, neon, argon, krypton, xenon, and radon. A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. Copper is in the ninth column of the transition metals in the d block of the fourth energy level of the periodic table. This would make the electron configuration for copper, 1s22s22p63s23p64s23d9 or in noble gas configuration [Ar] 4s23d9. However, because the 3d orbital is so much larger then.. Electron Configuration With Noble .
These are our P electrons because they're in P orbitals, and then once we're through our 2p6 electrons, we go to 3s2 and we have two more electrons, so it's 3p2. So that's the electron configuration for silicon. Now, we can write it out using noble .Chemistry questions and answers. 2. Based on the formula of the following molecules, write down the noble gas configuration of the cation (metal ion). (10 points) a Vanadium pentoxide (or divanadium pentoxide) is used in the steel industry and in ceramics. b. Copper (ll) chloride which is one of the salts used in fireworks is Copper (11) chloride.Write the condensed (noble-gas) electron configuration of copper. [He] [Ne] [Ar] [kr] Kr [Xe] [Rn] 1 2 3 4 5 6 क S pdf 0 0 0 1 00Noble gas is translated from the German noun Edelgas, first used in 1900 by Hugo Erdmann [5] to indicate their extremely low level of reactivity. The name makes an analogy to the term "noble metals", which also have low reactivity. The noble gases have also been referred to as inert gases, but this label is deprecated as many noble gas .K2Cr2O7 e. Fe2O3. 8. Based on the formula of the following molecules, write down the noble gas configuration of the cation (metal ion). (10 points) a. V2O5 b. CuCl2 c. KMnO4 d. K2Cr2O7 e. Fe2O3. There are 2 steps to solve this one.

Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Copper (Cu) [Ar] 3d 10 4s 1: 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1: 2, 8, 18, 1: 30: Electron configuration of Zinc (Zn) [Ar] 3d 10 4s 2: 1s 2 2s 2 2p 6 3s 2 .A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] for the 1s22s22p6 part of the configuration. Sodium's noble gas configuration becomes [Ne] 3s1.Write the noble gas electron configuration for copper, Cu: Like. 0. All replies. Answer. 1 year ago. Copper (Cu) is a d-block transition metal with an atomic number of 29. The complete electronic configuration of Cu can be written as follows- . A noble gas configuration of an atom consists of the elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons. So for sodium, we make the substitution of [Ne] [ Ne] for the 1s22s22p6 1 s 2 2 s 2 2 p 6 part of the configuration. Sodium's noble gas configuration becomes [Ne] 3s1 [ .
Dora (copyrighted as Dora: Say Hola to Adventure on Paramount+ and Dora and Friends on YouTube) is a computer-animated New Generation children's television series (set to stream exclusively on Paramount+[1] in the United States, and internationally it's set to stream day-and-date on Paramount+ and air on Nickelodeon channels in all markets .Enjoy online slots at the best slots casinos in 2024. Read on to discover various types of slot machines, play free slot games, and get expert tips on how to play online slots for real money!
noble gas configuration of copper|Noble gas configuration (video)